Classification / Names
Common names from other countries
Main reference
Size / Weight / Age
Max length : 50.0 cm TL male/unsexed; (Ref. 30578); common length : 35.0 cm TL male/unsexed; (Ref. 30578); common length :32 cm TL (female); max. published weight: 150.00 g (Ref. 40476); max. reported age: 10 years (Ref. 30578)
Length at first maturity
Lm 36.6, range 32 - 34 cm
Environment
Marine; freshwater; brackish; demersal; anadromous (Ref. 59043); depth range 10 - ? m
Climate / Range
Temperate; 5°C - 18°C (Ref. 12468), preferred ?; 69°N - 38°N, 11°W - 29°E (Ref. 59043)
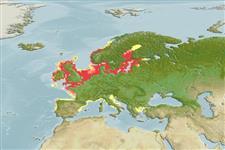
Distribution
Europe: southern Norway to France, including Ireland and the British Isles. Also in the Baltic Sea and along the French and western Italian coasts of the Mediterranean Sea (Ref. 59043). Absent from Black, Caspian and Polar seas (Ref. 3161). Landlocked populations from Lake Mjosa in Norway (Ref. 12269), Lakes Ladoga and Onega, upper Volga in Russia, Loch Lomond in Scotland, some Finnish lakes and possibly in Lough Neagh in Ireland (Ref. 59043). Appendix III of the Bern Convention (2002). Annex II (excluding Finnish and Swedish populations) and Annex V of the EC Habitats Directive (2007). Protected by law in the Netherlands (Ref. 12269).
Countries | FAO areas | Ecosystems | Occurrences | Introductions
Short description
Dorsal
spines
(total): 0;
Anal
spines: 0;
Anal
soft rays: 0. It is jawless with a round sucker-like mouth and has an outer circle of small teeth and an inner circle of large teeth (Ref. 88171). It has a typical eel-like shape with 2 dorsal fins and 7 gill openings behind the eye. It lacks paired fins. Young adults are uniformly greyish in colour. As it ages, the river lamprey becomes greenish-brown dorsally, golden yellow along the sides and white ventrally (Ref. 58137). In coastal waters of Germany, it can be confused with the sea lamprey (Petromyzon marinus), which is distinguished by having its teeth arranged in many consecutive circular rows (Ref. 88171). Other diagnostic features: Adults 8.6-49.2 cm TL. Body wet weight in individuals 18.0-49.2 cm TL, 30-150 g. Body proportions, as percentage of TL (based on 48 specimens measuring 10.8-38.6 cm TL): prebranchial length, 10.0-12.9; branchial length, 7.9-11.3; trunk length, 46.2-54.3; tail length, 24.1-30.3; eye length, 1.4-3.1; disc length, 4.6-7.0. Urogenital papilla length, as a percentage of branchial length, in 19 spawning males measuring 19.7-28.3 cm TL, 15.9-37.5. Trunk myomeres, 58-66. Dentition: marginals, 70-95; supraoral lamina, 2 unicuspid teeth; infraoral lamina, 5-9 either all unicuspid teeth or, more frequently, the lateralmost are bicuspid and the internal ones unicuspid; 3 endolaterals on each side; endolateral formula, typically 2-3-2, rarely 1-3-2 or 2-3-1; 1-2 rows of anterials; first row of anterials, 4-7 unicuspid teeth; exolaterals absent; posterials absent; transverse lingual lamina, 8-18, usually 12-14, unicuspid laminae straight or parentheses-shaped and each with 9-13 unicuspid teeth. Marginal membrane present. Velar tentacles, 4-10, with tubercles; no velar wings. Body coloration in recently metamorphosed individuals silvery; in preserved upstream migrants, bluish brown or lead gray on the dorsal aspect tending towards silvery on the lateral aspects and whitish or yellowish on ventral aspect. Early upstream spawning migrants returning from the sea have a bronze sheen. Dorsal fins of maturing individuals may have a purplish tint. Iris is golden yellow. Body coloration in the landlocked population in Lake Ladoga is completely black. Lateral line neuromasts unpigmented or darkly pigmented. Extent of caudal fin pigmentation, absent or trace in young adults and 75% or more in spawning individuals. Caudal fin shape, spade-like. Oral fimbriae, 84-112. Oral papillae, 11-20 (Ref. 89241).
IUCN Red List Status (Ref. 115185)
Threat to humans
Poisonous to eat (Ref. 5504)
Human uses
Fisheries: minor commercial; bait: usually
Tools
Special reports
Download XML
Internet sources
Estimates of some properties based on models
Phylogenetic diversity index
PD50 = 0.5005 many relatives (e.g. carps) 0.5 - 2.0 few relatives (e.g. lungfishes)
Trophic Level
4.5 ±0.80 se; Based on food items.
Resilience
Medium, minimum population doubling time 1.4 - 4.4 years (tm=4-6; tmax=10; Fec = 4,000)
Vulnerability
High vulnerability (62 of 100)
Price category